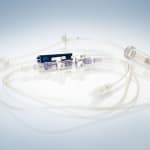
 Phillips-Medisize Corporation will be presenting versatile solutions for plastics products in the sectors medical technology and diagnostics at MEDTEC in Stuttgart, Germany, between March 13 and 15, 2012.
Phillips-Medisize Corporation will be presenting versatile solutions for plastics products in the sectors medical technology and diagnostics at MEDTEC in Stuttgart, Germany, between March 13 and 15, 2012. The company will be demonstrating a wide range of products from peristaltic pumps, titration plates and mixing-injectors to complete sets for MDD-applications, for instance for eye contact rinses. Phillips-Medisize convinces customers by presenting the complete value chain, from the initial idea to the finished product, from the design to the medical product in sterile packaging, ready to be used: The company’s market and strength are complex disposables in particular, controlled throughout by highly prioritized quality assurance according to ISO 13485 and FDA-Standards or by CGMP (current good manufacturing practice).
Phillips-Medisize concentrates on four market segments: medical & diagnostic devices, drug delivery devices, primary pharmaceutical packaging, and airway management. Klaus Schmid, Business Development Manager at Phillips-Medisize emphasizes: “We encourage discussions of design and functionality with our customers, based on partnership. We can create product prototypes in our in-house mould making facility in Finland. The project will enter the injection moulding process and on to assembly only when everything is perfect and to the customer’s full satisfaction – in clean-room conditions class 7/8.“
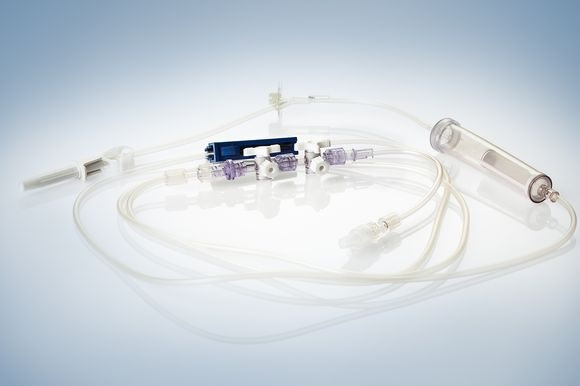
Among other exhibits, Phillips-Medisize will show a peristaltic pump, where a tube is being actuated by contraction, to inject, for instance, a contrast medium into a patient’s vein. The industrialization process took place within the company, that is from the prototype right through to the injection molded and ultra sound hermetically sealed pump. Other exhibits include a bone cement mixing gun, complete transfer systems including tube sets. In the sector disposable diagnostic devices, Phillips-Medisize will exhibit a titration plate with 1536 bores for polymerase chain reaction in DNA replication (PCR).
Consistent fluorescence of the base material has to be guaranteed here. Other important factors are the heatability of the plate at a high stability rate as well as an accuracy in the lower range of one hundreds of a millimeter. “These characteristics, as sought by the customer, have been developed specifically for this product. That is what gives Phillips-Medisize its unique market positioning“, reiterates Klaus Schmid. In the sector diagnostics, Phillips-Medisize also produces reaction tubes to be used, for example, for blood tests and single-direction sets for point of care diagnostics.
A strong research and development department and several Business Development Managers in the Medical & Diagnostic Devices division emphasize the high ranking of this sector within the entire organization. Quality control and quality assurance are of essential importance with products in the medical technology field: Phillips-Medisize has the appropriate in-house control facilities to check and test all products and also uses cytotoxicity tests, bioburden testing, LAL or risk analysis through FMEA. Validation of the processes follows DQ-, IQ-, OQ- und PQ procedures.


